OBJECTIVE. The purpose of this study was to retrospectively
evaluate the use of sonography as the primary imaging modality for congenital
hypothyroidism (CH).
MATERIALS AND METHODS. From our regional registry, we reviewed the
cases of patients for whom either sonography or 99mTc-pertechnetate scanning was performed for CH
between 2003 and 2010. Ultrasound studies were reviewed for presence, size,
echotexture, vascularity, and location of the thyroid gland. Technetium-99m-pertechnetate
scans were evaluated for the presence and location of the thyroid gland. The
ultrasound studies were compared with the 99mTc-pertechnetate scans. We assessed the use of
ultrasound as the primary imaging modality for the evaluation of
RESULTS. We identified the cases of 124 patients (89
girls, 35 boys). Ultrasound studies were available for 121 patients, and 99mTc-pertechnetate studies for 62 patients. Three
patients were examined only by 99mTc-pertechnetate
scanning. The final imaging results were normal location with normal size or
diffuse enlargement of the thyroid gland (n = 47), sublingual thyroid
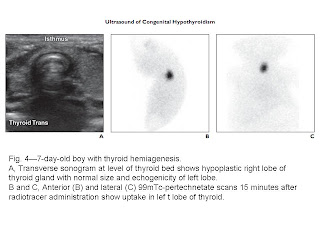
gland (n = 49), agenesis (n = 18), hypoplasia (n = 8), and
hemiagenesis (n = 2). Compared with 99mTc-pertechnetate scanning, ultrasound had high
(100%) specificity and low (44%) sensitivity for detection of sublingual
thyroid gland.
CONCLUSION. We suggest using ultrasound as the primary
imaging modality for guiding the treatment of children with CH, potentially
decreasing radiation exposure and cost.
Congenital
hypothyroidism (CH) is defined as thyroid hormone deficiency present at birth.
It can be subdivided into permanent and transient types. Permanent CH refers to
persistent deficiency of thyroid hormone that requires lifelong treatment [1]. Transient CH
refers to a temporary deficiency of thyroid hormone. The deficiency is present
at birth, but recovery to normal thyroid hormone production usually occurs within
the first few months or years of life.
Almost all
neonates are screened for CH.
In undeveloped
countries, the most common cause of CH is iodine deficiency (transient CH), but
in the developed world, 85% of cases of CH are caused by thyroid dysgenesis
(aplasia, hypoplasia, or ectopia). Inborn errors of thyroid hormone
biosynthesis (dyshormonogenesis) or defects in peripheral thyroid hormone
transport, metabolism, or action account for 10–15% of cases and are also
associated with genetic defects. Secondary, or central, CH may occur with
isolated TSH deficiency, but more commonly it is associated with congenital
hypopituitarism [1].
Determining the
cause of CH guides management and genetic consultation because it has
prognostic implications [1]. Although
thyroid hormone replacement is the initial treatment in all cases, if the
patient has a normal-appearing eutopic thyroid gland, a trial of discontinuing
levothyroxine when the patient is approximately 3 years old is often undertaken
to differentiate permanent from transient CH. If the thyroid gland adequately
functions, no further replacement hormone is required. If no thyroid tissue is
found or if dyshormonogenesis has occurred, the child needs thyroid
supplementation for life.
Imaging studies
to help determine the underlying cause of CH include thyroid radionuclide
examinations and thyroid ultrasound. Thyroid radionuclide studies with 99mTc-pertechnetate or 123I are considered the standard for imaging in
the evaluation of thyroid dysgenesis. Although 99mTc-pertechnetate is preferred because of lower
thyroid and total body radiation dose (≈ 0.04 mSv compared with 0.35 mSv) [7], both result in
radiation exposure to the patient. In the case of eutopic location of the
thyroid gland, an 123I uptake followed
by a 99mTc-pertechnetate perchlorate discharge test is
the definitive study for identifying an organification defect of the thyroid
gland [8].
Sonography does
not involve the risk of ionizing radiation and can be used to differentiate
thyroid dysgenesis and other causes of CH in which the thyroid gland has normal
morphologic features [9, 10]. Sonography,
however, has lower sensitivity than 99mTc-pertechnetate scintigraphy in the detection of sublingual thyroid.
The use of color Doppler ultrasound (CDUS), however, has been found to increase
the detection of sublingual ectopic thyroid [1, 2, 5, 11].
For several years
at our facility, we have been using sonography as the primary screening imaging
modality in the care of patients with CH and using 99mTc-pertechnetate scintigraphy primarily for
patients with thyroid dysgenesis. In this study, we summarize the experience
with the use of ultrasound in CH that led us to recommend using an
ultrasound-based imaging algorithm [12, 13].
Materials and Methods
Patients
A retrospective
review was performed of the cases of all patients whose condition was diagnosed
as CH at our institution between January 1, 2003, and December 31, 2010. Only
patients whose thyroid ultrasound or 99mTc-pertechnetate scans were available for
review were included. Institutional review board approval was obtained with a
waiver of informed consent for the study. All but three CH patients were
initially imaged with thyroid ultrasound. The decision to order a 99mTc-pertechnetate scan was then made by an
endocrinologist on the basis of the ultrasound results. Typically 99mTc-pertechnetate scanning was performed to
evaluate or confirm ectopic sublingual thyroid when ultrasound showed thyroid
dysgenesis.
TABLE 1:Reference Standard for Thyroid Size (cm) by
Age
TABLE 2:Causes of Congenital Hypothyroidism in 124
Patients Between 2003 and 2010
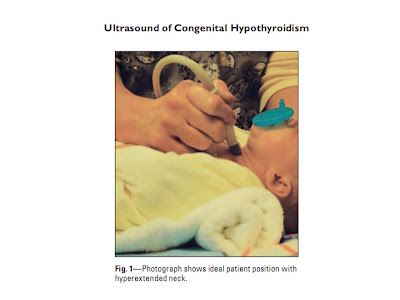
Fig. 1:Photograph shows ideal patient position with hyperextended neck.
Imaging Technique
For thyroid
sonography, all patients were examined in the supine position with the neck
hyperextended by placement of a folded towel beneath the scapula (Fig. 1). A 7–15 MHz
linear transducer with a small footprint was used (Acuson Sequoia 512, Siemens
Healthcare, or HDI 5000 IU 22, Philips Healthcare). Gray-scale transverse and
longitudinal images were obtained from the base of the tongue. CDUS was
performed in some patients to better depict ectopic sublingual thyroid.
For 99mTc-pertechnetate scintigraphy, the scan was
performed with 1–2 mCi of 99mTc-pertechnetate
IV (dose calculated on basis of patient’s weight). Images were obtained in the
anterior and lateral views 15 minutes after administration.
Imaging Evaluation and Data Analysis
All of the
imaging studies were reviewed at our standard clinical PACS workstation
(Synapse, Fujifilm). Both ultrasound and 99mTc-pertechnetate scans were separately and
independently reviewed by a pediatric radiologist (fellowship trained with 5
years of experience) and a nuclear medicine physician (30 years of experience).
Ultrasound
studies were reviewed for the presence (eutopic, ectopic, or agenesis) of
thyroid tissue, size (normal, hypoplastic, or hyperplastic) compared with the
reference standard (Table 1) [14], echotexture
(normal or increased echogenicity), and degree of thyroid vascularity (normal,
increased, decreased). Technetium-99m-pertechnetate scans were evaluated for
the presence (eutopic, ectopic or agenesis) of thyroid tissue and subjective
degree (normal, increased, or decreased) of radiotracer uptake.
We used
descriptive statistical analysis for each modality, divided into eutopic
location, ectopic location, and agenesis of the thyroid gland. We also compared
the sensitivity, specificity, and accuracy of the modalities using 99mTc scintigraphic results (when available) as
the reference standard. On ultrasound images we evaluated the presence,
location, size, echotexture, and vascularity of thyroid gland, and on the 99mTc-pertechnetate studies—the reference standard
for evaluation of sublingual thyroid— we evaluated presence, location, size,
and uptake.
Discussion
The treatment of
CH patients is empiric and not guided by imaging findings. A neonate with a
diagnosis of CH is immediately treated with thyroid hormone replacement [15]. Using a higher
starting dose to more quickly normalize TSH levels to the target range within 2
weeks to normalized developmental IQ even in patients with severe CH is the
main purpose of treatment. The initial thyroid hormone (levothyroxine) dose for
eutopic thyroid gland is approximately 10 μg/kg/d, compared with 15 μg/kg/d for
noneutopic thyroid gland [16].
Permanent CH can
be assumed if ultrasound or radionuclide imaging shows the thyroid gland is
absent or ectopic (together referred to as dysgenesis) or if at any time during
the first year of life, the serum TSH concentration rises above 20 mU/L owing
to undertreatment. The American
Academy
Thyroid
scintigraphy is considered the reference standard for the evaluation of CH.
In our 7-year
cohort of patients with CH, we found a higher frequency of eutopic thyroid
gland than reported in the literature. This finding may be related to a higher
percentage (36%) of transient hypothyroidism in our screening program [8]. Regardless of
the underlying cause, the initial treatment of CH is the same. However,
patients with eutopic thyroid gland may need further investigation to
differentiate between the permanent and transient forms of CH.
The reported
incidence of primary hypothyroidism has increased in the United States
Several investigators
who used both scintigraphy and ultrasound for the diagnosis of CH have
recommended ultrasound as a first-line study to avoid radiation associated with
scintigraphy [17, 20, 21]. We are the
first, to our knowledge, to report experience using thyroid ultrasound as the
primary imaging evaluation of CH with selective use of scintigraphy in children
with thyroid dysgenesis.
The main
limitation of ultrasound is decreased sensitivity in the evaluation of ectopic
thyroid gland. The ultrasound diagnosis of ectopic thyroid gland depends on
technique and the experience of the sonographer. Marked variation in
sensitivity (0–80%) has been reported among medical centers [1–3, 5, 11, 18, 19]. The
sensitivity of sonography in the detection of ectopic thyroid gland in our
series was 44%. Using CDUS increases the sensitivity of diagnosis of ectopic
thyroid gland [1, 2, 5, 11]. In our series,
in most cases of missed ectopic thyroid gland, CDUS was not used. The reported
specificity of ultrasound in the detection of ectopic thyroid gland is high [1–3, 5, 11, 18, 19, 21]. In our series,
the specificity of sonography was 100%.
Our experience
showed that when ultrasound depicts ectopic or eutopic thyroid gland, the
scintigraphic results will not change the initial management. For precise
diagnosis of agenesis versus sublingual thyroid gland in all patients with
ectopic thyroid gland, scintigraphy can be used selectively when ultrasound
does not depict any thyroid tissue. In our series, that would have obviated
scintigraphy for 54% of the patients.
For management
guidance, it is important to differentiate patients with eutopic thyroid gland
from those with thyroid dysgenesis. Patients with thyroid dysgenesis are being
treated for life with thyroid hormone replacement. The ectopic thyroid gland
eventually involutes owing to suppression of TSH. Differentiation between
thyroid agenesis and ectopic thyroid gland does not change management.
Scintigraphy can therefore be used selectively only in cases of equivocal
ultrasound findings, such as hypoplastic thyroid gland. In our series, we did
not perform scintigraphy for most patients with eutopic thyroid gland and
therefore do not have a correlation with thyroid size or parenchymal
echotexture. With this management, we would remove the need for scintigraphy
for 90% of patients. This approach will save both radiation and cost with no
change in management.
Imaging of
patients with CH has a role in the evaluation of the cause, in prognosis, and
in guiding management. Ultrasound of the thyroid can be used to differentiate
patients with thyroid dysgenesis from patients with eutopic thyroid. Thyroid
dysgenesis is typically a sporadic disorder and carries no recurrence risk of
CH with future pregnancies. Patients with eutopic thyroid gland are a
heterogeneous group; some have a risk of recurrence in future pregnancies.
Genetic consultation can be considered [1].
Our study had
several limitations. First, our study was performed as a retrospective review
of imaging findings, and there was inconsistent use of CDUS, possibly
decreasing sensitivity in the detection of ectopic sublingual thyroid gland.
Second, the ultrasound and 99mTc studies were
reviewed by a single radiologist, possibly biasing interpretation of the
studies. However, compared with original reports, in only two studies (3%) did
the retrospective evaluations vary. Because the studies were reviewed by a
single radiologist, we could not assess interobserver variability. Third, we
imaged only patients who were evaluated prenatally at our institute. Fourth,
the study did not include follow-up on euthyroid patients. However, the
incidence of transient hypothyroidism in our institution (36%) had been
published [8].
Conclusion
Ultrasound can be
used as the primary imaging modality for guiding treatment of children with CH,
potentially decreasing radiation exposure and cost. Scintigraphy can be
reserved for the few patients with equivocal ultrasound findings, such as
hypoplastic thyroid gland.
AJR:199, September 2012








Không có nhận xét nào :
Đăng nhận xét