
Clinical Context
The National Cancer Institute estimates that approximately 40% of women undergoing screening mammography have dense breast tissue. High breast density hinders mammographic interpretation, which may delay detection of breast cancer until it is at a more advanced and difficult-to-treat stage.Less-dense breasts have a high amount of fatty tissue, whereas dense breasts have a high amount of connective and fibroglandular tissue. Breast cancer and fibroglandular breast tissue both appear as solid white areas on mammograms, rendering interpretation more difficult.
Study Synopsis and Perspective
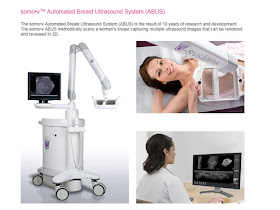
The US Food and Drug Administration (FDA) approved the first ultrasound device for use in combination with mammography in women with dense breast tissue.The device, known as the somo-v Automated Breast Ultrasound System (ABUS), provides clinicians with an additional resource in screening women with dense breasts. The indication is limited to use in women who have a negative mammogram result and no symptoms of breast cancer.
Mammograms of dense breasts can be difficult to interpret, the FDA points out in its announcement of the approval.
"A physician may recommend additional screening using ultrasound, for women with dense breast tissue and a negative mammogram," said Alberto Gutierrez, PhD, director of the Office of In Vitro Diagnostic Device Evaluation and Safety at the FDA's Center for Devices and Radiological Health. "The somo-v ABUS is a safe and effective breast ultrasound tool when such screening is recommended."
Approximately 40% of women undergoing screening mammography have dense breasts, according to National Cancer Institute estimates. These women have an increased risk for breast cancer, with detection usually at a more advanced and difficult-to-treat stage, the FDA said.
In April, an expert advisory committee of the FDA voted unanimously to recommend the expanded use of the ultrasound device as a screening tool for women with dense breast tissue.
However, as reported by Medscape Medical News, some of the panel members had concerns about the device because of its automated nature. In contrast to handheld ultrasound, medical specialists who operate an automated ultrasound do not have to be imaging experts, Robert Faulk, MD, from Medical Imaging Consultants in Omaha, Nebraska, noted during the meeting. "For instance, obstetricians and gynecologists could do ABUS and interpret the results themselves, along with mammography findings," Dr. Faulk said. "That could have a deleterious effect on healthcare for many women," he added.
"ABUS as a screening tool could potentially be applied to 40 million women in the United States [with dense breasts], and if it were used by nonimaging specialists, the false-positive rates could go through the ceiling," said Daniel Kopans, MD, from Harvard Medical School in Boston, Massachusetts. Dr. Kopans commented that postmarket surveys of the false-positive rate from the new ultrasound device could provide important data on the effect of its use as a screening tool in women with dense breasts.
Dense breasts have a high amount of connective and fibroglandular tissue compared with less-dense breasts, which have a high amount of fatty tissue, the FDA explained.
Fibroglandular breast tissue and tumors both appear as solid white areas on mammograms, which can complicate interpretation. Dense breast tissue may obscure smaller tumors, potentially delaying detection of breast cancer, according to the FDA.
Ultrasound imaging has been proven capable of detecting small masses in dense breasts.
A clinical study has shown a statistically significant increase in breast cancer detection when images generated by this new device were reviewed in conjunction with mammograms compared with mammograms alone. The study involved board-certified radiologists who reviewed mammograms alone or in conjunction with device-generated images for 200 women with dense breasts and negative mammograms.
As part of the approval, the FDA requires that the manufacturer train clinicians and technologists using the new ultrasound device, and that the manufacturer provide each facility with a manual clearly defining system tests required for initial, periodic, and yearly quality-control measures.
The ultrasound works via a transducer that directs high-frequency sound waves at the breast. The specially shaped transducer of the device can automatically scan the entire breast in approximately 1 minute to produce several images for review, according to the FDA press materials.
More information on the new ultrasound device is available on the FDA Web site.
Clinical Implications
- The FDA has approved a new automated ultrasound device to be used in combination with mammography in women with dense breast tissue. This is the first ultrasound device approved for this indication. The specially shaped transducer of the device directs high-frequency sound waves at the breast to scan the entire breast in approximately 1 minute, automatically producing several images for review.
- The FDA has approved the ultrasound device for use only in women with negative mammography results and no symptoms of breast cancer. In these women, the device is a safe and effective breast ultrasound tool. Compared with mammograms alone, mammograms plus images generated by the new device interpreted by board-certified radiologists were associated with a statistically significant increase in breast cancer detection.
- Unlike handheld ultrasound, automated ultrasound does not have to be operated by an imaging expert. This may be a potential drawback in that medical practitioners without specialized radiographic training could use the new device and interpret the results themselves, along with mammography findings. This could dramatically increase false-positive rates. The FDA is requiring that the manufacturer train clinicians and technologists using the new ultrasound device, and that each facility is given a manual clearly defining system tests required for quality control.
Abstract: The idea of
an automated whole breast ultrasound was developed three decades ago. We
present our initial experiences with the latest technical advance in this
technique, the automated breast volume scanner (ABVS) ACUSON S2000TM.
Volume data sets were collected from 50 patients and a database containing 23
women with no detectable lesions in conventional ultrasound (BI-RADS®-US
1), 13 women with clearly benign lesions (BI-RADS®-US 2), and 14
women with known breast cancer (BI-RADS®-US 5) was created. An
independent examiner evaluated the ABVS data on a separate workstation without
any prior knowledge of the patients’ histories. The diagnostic accuracy for the
experimental ABVS was 66.0% (95% confidence interval [CI]: 52.9–79.1). The
independent examiner detected all breast cancers in the volume data resulting
in a calculated sensitivity of 100% in the described setting (95% CI:
73.2%–100%). After the ABVS examination, there were a high number of requests
for second-look ultrasounds in 47% (95% CI: 30.9–63.5) of the healthy women
(with either a clearly benign lesion or no breast lesions at all in
conventional handheld ultrasound). Therefore, the specificity remained at 52.8%
(95% CI: 35.7–69.2). When comparing the concordance of the ABVS with the gold
standard (conventional handheld ultrasound), Cohen’s Kappa value as an
estimation of the inter-rater reliability was κ = 0.37, indicating fair
agreement. In conclusion, the ABVS must still be regarded as an experimental
technique for breast ultrasound, which definitely needs to undergo further
evaluation studies.
Keywords: breast cancer, automated breast ultrasound, automated breast volume scanner, ABVS
Keywords: breast cancer, automated breast ultrasound, automated breast volume scanner, ABVS



Không có nhận xét nào:
Đăng nhận xét