Sonographically
Guided Lavage Aspiration Technique
The patient is positioned such that the calcification to be
treated is well visualized and amenable to needle placement and that both the
patient and physician will be comfortable throughout the procedure. Depending
on the
calcification location, particularly within the supraspinatus
tendon and deep to the overlying acromion process of the scapula, it may be
necessary to experiment with various armpositions of the patient to achieve
this goal. Generally, the procedure is performed with the patient in the
lateral decubitus or supine position for supraspinatus and
infraspinatus calcification and in the supine position if the subscapularis
tendon is the target.
A high-resolution linear probe (10–17 MHz) is used for this
procedure, as the target calcification is usually very superficial. A 25-gauge
needle is used for local anesthesia, and a 16- to 18-gauge needle is generally
used for the lavage aspiration. Although some authors have advocated a smaller needle size (22 gauge), 20 in this author’s experience, a
larger needle allows a faster andmore complete evacuation of calcium. Although
others also advocate a 2-needle approach, and excellent results have been
achieved with this method, this author has not found that approach to
substantially improve calcium removal or to decrease the procedure time.
A recent study of 462 patients suggests that warming the
lavage fluid may help improve dissolution of calcium and shorten the procedure
time, which may prove to further optimize this technique.
However, this large study did not show a significant
difference in patient outcomes as determined by visual analog scale scores
between the two groups.
In preparation for the lavage aspiration, a series of syringes
are prepared (3–6, depending on the size of the calcification) containing a
mixture of saline and an anesthetic. This author favors a blend of 70% sterile
saline and 30% lidocaine, 1%. A syringe containing an additional anesthetic
(0.25% bupivacaine HCl) and cortisone (triamcinolone acetonide or methylprednisolone
acetate) is also prepared for the subacromial-subdeltoid bursal injection that
concludes the procedure, with the bupivacaine providing relief of postprocedure discomfort for several hours.
Continuous sonographic visualization of the calcification
and needle is necessary throughout the procedure.
A liberal amount of anesthetic is administered
subcutaneously, within the deeper soft tissues and within the
subacromial-subdeltoid bursa, being sure that no air is introduced into the
soft tissues or adjacent subacromial-subdeltoid bursa, extending from the skin entry site to
themargin of the calcification along the expected path of lavage needle
placement. If air is injected and is superficial to the target calcification,
particularly within the subacromial-subdeltoid bursa, the calcification may be
entirely obscured, and the proceduremay need to be postponed until the air is
resorbed, which may take several days. With appropriate local anesthetic
administration, the procedure is generally well tolerated with only mild
discomfort. With continuous sonographic visualization, the 16- or 18-gauge
needle is advanced into the epicenter of the calcific focus with a single
puncture (Figure 3). Using the syringes filled with the
anesthetic/saline mixture noted above,
intermittent plunger pressure and release are performed until a cavity
forms within the focus of calcification (Figure 4). At this point, swirling of
echogenic material (calcium) will be seen within the cavity, and with plunger
release, this calcific material will decompress into the syringe. If more than
a single puncture is made into the lesion, the lavage material may decompress
through this additional hole in the calcific focus, and the yield of calcium removed will
be diminished.
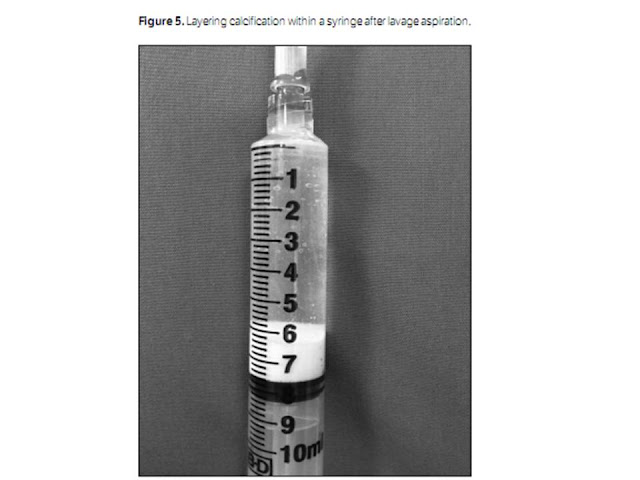
As large amounts of calcium fill the syringe, exchange is
made for new clear syringes until no further calcification may be removed. The
calcific material removed will be seen to layer within the dependent portion of
the syringe (Figure 5). At this point in the procedure, any remaining
calcification along the wall of the original focus is fenestrated using the
needle. If additional foci of calcification are present, these are treated in
the same manner. At the conclusion of this process, any remaining calcium
fragments too small for lavage are also fenestrated with the needle. Finally,
the mixture of anesthetic and corticosteroid described above is injected into
the adjacent subacromial-subdeltoid bursa, which will provide considerable pain
relief over the next several weeks to months as additional calcific material
decompresses into the bursa from the involved rotator cuff tendon.
Although follow-up radiographs are not routinely obtained,
they may show a rapid and marked decrease in the amount of calcification
remaining within the tendon. In patients with recurrent or residual
pain after therapy, subsequent sonographic examinations may be
performed to assess the degree of calcification within the tendon or
subacromial-subdeltoid bursa. Patients in this group may often be effectively
treated with a sonographically guided bursal cortisone and anesthetic
injection.
Conclusions
Calcific tendinosis of the rotator cuff is a commonly
diagnosed entity that is responsible for a great deal of patient pain and
limitation of mobility. Although radiographs remain the mainstay of initial
calcium visualization and diagnosis, sonography can localize the calcification
to the specific tendon involved, assess the entire rotator cuff for tears or tendinosis,
and also evaluate the adjacent biceps tendon and subacromial-subdeltoid bursa
for concomitant abnormalities. Finally, diagnostic sonography provides the means by which this
condition can be safely treated by the percutaneous technique described above.
This technique quickly removes and fragments the problematic calcification with
a low incidence of complications, and multiple studies have shown an excellent
clinical response in most patients. Lavage aspiration with sonographic guidance
has thus become the optimal modality for effective treatment of this painful condition.